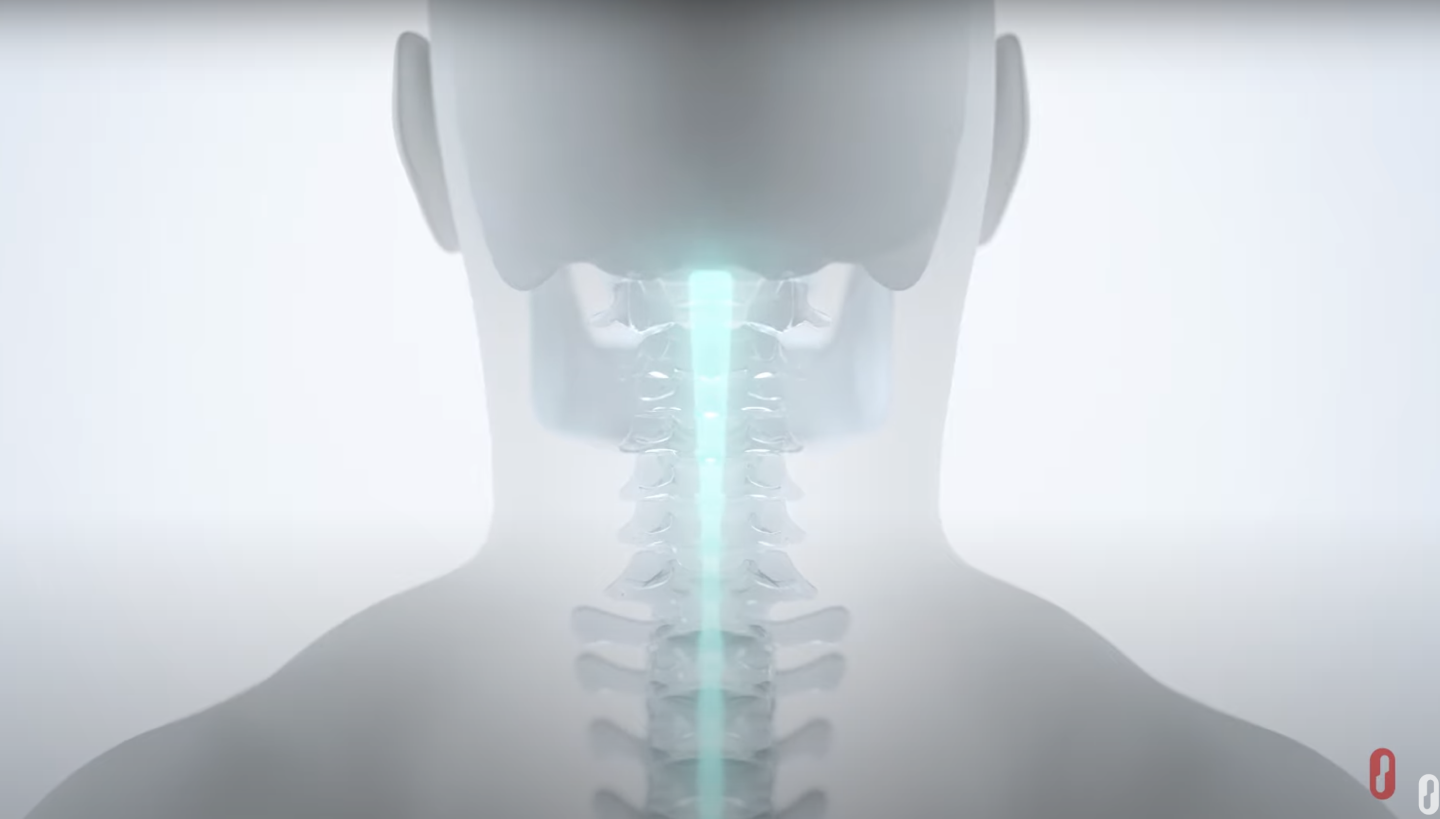
Our
Technologies
External and implantable neuromodulation platforms built to deliver ARC Therapy.
Our Mission
Driven to restore
Movement
Function
Independence
in people with spinal cord injury
Join our Patient Registry if you are interested in our clinical trials
Discover our Therapy
Discover our Therapy
External Platform
External Platform
Implantable Platform
Implantable Platform
Implantable Platform
Implantable Platform
Read our Publications
Please complete this form to
contact us or receive updates
The ONWARD® Medical ARCEX® System is cleared for use only in the United States. ONWARD ARCIM® and ARCBCI™, alone or in combination with a brain-computer interface (BCI), are investigational and not available for commercial use.
*The ARCEX System is intended to deliver programmed, transcutaneous electrical spinal cord stimulation in conjunction with functional task practice in the clinic to improve hand sensation and strength in individuals between 18 and 75 years old that present with a chronic, non-progressive neurological deficit resulting from an incomplete spinal cord injury (C2-C8 inclusive).